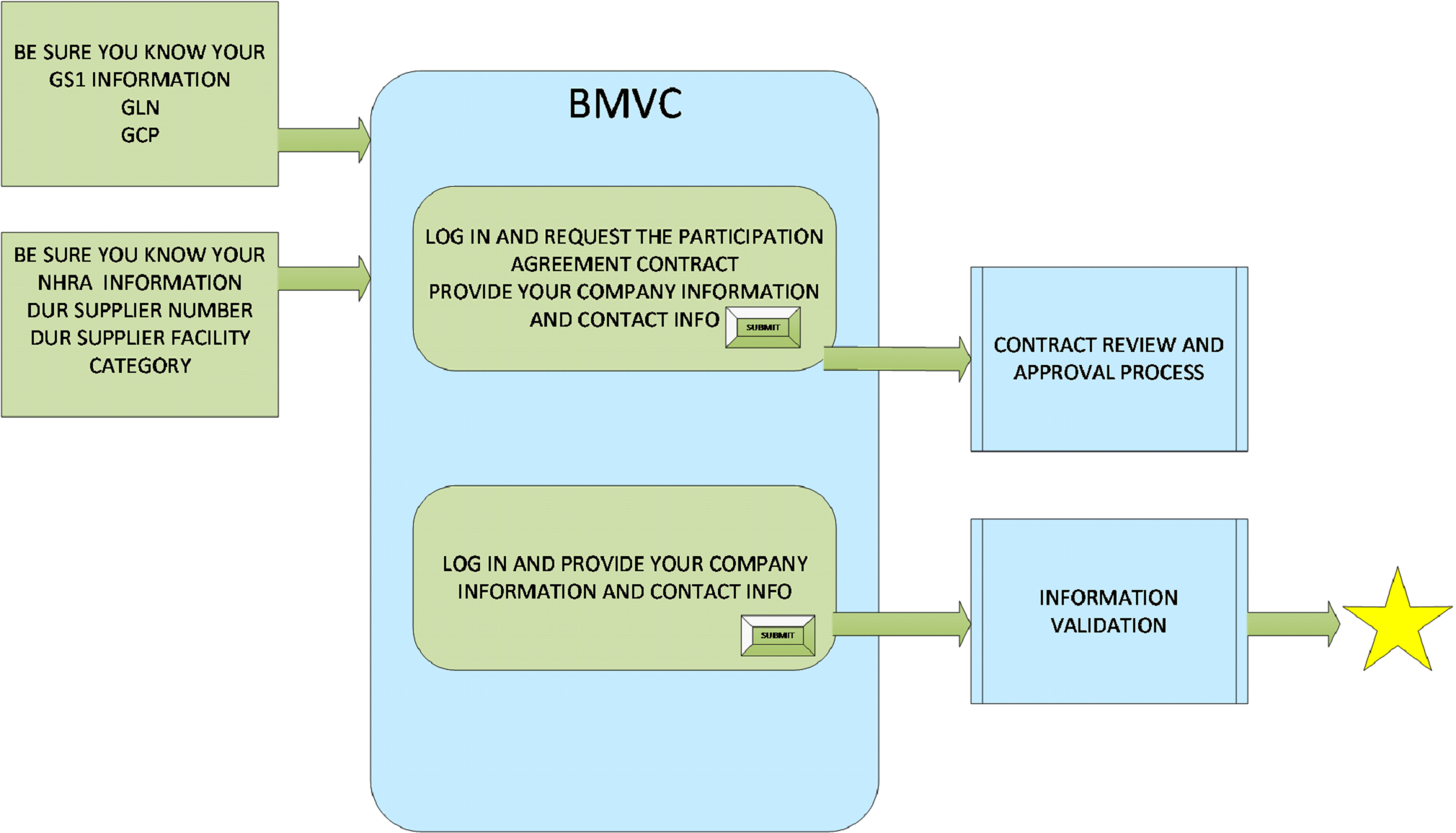
There are 4 steps for the Dispenser to register and be able to submit data to the NHRA’s BMVC Traceability Hub. The first three steps are pre-requisites to the BMVC Traceability Hub registration process for Dispensers.
All Dispensers must be registered with GS1 and have a Global Location Number (GLN) and Global Company Prefix (GCP) assigned. Make sure you know the GS1 Global Location Number (GLN) and Global Company Prefix (GCP) assigned to this Dispenser.
In addition to registering with GS1, registration applications must be submitted and approved by the NHRA to permit pharmaceutical products to be sold in Bahrain. Payment must be made to the NHRA at the time of registration. The NHRA registration process contains all of the necessary Dispenser location information.
All Dispensers need to complete these registrations with the NHRA and have the Supplier Identification information for their organization ready when they register with BMVC Traceability Hub.
All MAH’s, Distributors / Wholesalers and Dispensers must complete a Participation Agreement contract with the NHRA’S BMVC Traceability Hub before the MAH registration can be completed.
You will need to provide all required information to identify your organization with NHRA’S BMVC Traceability Hub. After this information is submitted, you will be contacted by NHRA BMVC Traceability Hub staff to complete the Participation Agreement. The Participation Agreement is mandatory for all Dispensers and is non-negotiable.
| Information Needed | Comments |
|---|---|
| Company Name | As shown in GS1 and NHRA sites |
| Company Address | As shown in GS1 and NHRA sites |
| City | As shown in GS1 and NHRA sites |
| State | As shown in GS1 and NHRA sites |
| Zip Code / Postal Code | As shown in GS1 and NHRA sites |
| Country | As shown in GS1 and NHRA sites |
| Time Zone | Time Zone for the Dispenser location |
| Type of Dispenser |
Based on the DUR Supplier Facility Category
|
| Supplier Facility Number | This is the DUR Supplier Number |
| Name of the Administrative Contact | The person who will sign the Participation Agreement |
| E-mail address for the Administrative Contact | The Company issued email address for the person designated as the Administrative Contact |
| Phone Number for the Administrative Contact | The Country Code plus phone number for the person designated as the Administrative Contact |
| Number of locations Registered with NHRA | The number of unique pharmacy sites your company uses to dispense the medicines registered with the National Health Regulatory Authority. |
The person the Dispenser identifies as Administrative Contact must have the authority to execute a contractual agreement on behalf of the Dispenser.
Once the Dispenser has completed its NHRA and GS1 registrations, the Administrative Contact must select Registration Link for the Dispenser Registration. Your BMVC Traceability Hub Participation Agreement contract (STEP 3 above) must be fully executed before the registration will be finalized by NHRA.
| Information Needed | Comments |
|---|---|
| Company Name | As shown in GS1 and NHRA sites |
| Company Address | As shown in GS1 and NHRA sites |
| City | As shown in GS1 and NHRA sites |
| State | As shown in GS1 and NHRA sites |
| Zip Code / Postal Code | As shown in GS1 and NHRA sites |
| Country | As shown in GS1 and NHRA sites |
| Time Zone | Time Zone for the Dispenser location |
| Name of the Administrative Contact | The person who will sign the Participation Agreement and handle financial payment |
| E-mail address for the Administrative Contact | The Company issued email address for the person designated as the Administrative Contact |
| Phone Number for the Administrative Contact | The Country Code plus phone number for the person designated as the Administrative Contact |
| BMVC Participation Agreement Contact Number | Approved Agreement Number you receive from the BMVC |
| GS1 Global Location Number | As shown in GS1 look-up site |
| GS1 Company Prefix | As shown in GS1 look-up site |
| Supplier Facility Number | As shown in NHRA sites |
| VAT # | Provided by your Company |
| Name of your company Technical Point of Contact (POC) | The person who will act as the Point of Contact (POC) for the technical onboarding of the Dispenser |
| E-mail address for the Technical Point of Contact | The Company issued email address for the Point of Contact (POC) for the technical onboarding of the Dispenser |
| Phone Number for the Technical Point of Contact | The Country Code plus phone number for the Point of Contact (POC) for the technical onboarding of the Dispenser |
| Number of Dispenser locations Registered with NHRA | The number of unique disperser locations your company uses to store and dispense medicines registered with the National Health Regulatory Authority. |
After you have submitted your Dispenser information and the required documentation, the NHRA’s BMVC Administrative team will verify your company and confirm the Dispenser and its intended products are in good standing with the PPR.
With confirmation successfully processed, the Administrative Contact and the Technical Point of Contact will receive an email stating the registration is complete and the technical testing will be initiated.