Sign up with NHRA-MVC is required to use the Traceability Hub. All Invoicing Companies (MAHs, Manufacturers), Agents/Distributors (Importers), Dispensers (Hospitals, Pharmacies, Clinics) will need to sign up.
You can sign up on our website at www.NHRA-MVC.bh
The Supreme Council of Health (SCH) and the National Health Regulatory Authority (NHRA) believe that global standards for automatic identification provide an opportunity to make the healthcare supply chain in the Kingdom of Bahrain safer as well as more efficient and accurate.
The NHRA-MVC Traceability Hub is part of the NHRA program committed to enhancing the lives of the people of Bahrain. For further information regarding this effort please refer to the following:
The requirement to submit your product information to the BrandSync Portal is part of the Medicines Barcoding and Serialization Guideline that was published by the National Health Regulatory Authority (NHRA) in May, 2021.
The NHRA-MVC Traceability Hub is for tracking all registered medicines; from the time they are shipped to Kingdom of Bahrain, to the point they are dispensed to patients.
The most important implementation resource for barcode identification are the over 100 GS1 Member Organizations in countries all around the world. There are ten Steps to Barcode Implementation
This GS1 guide takes new barcode users through the basic steps they must take to begin using barcodes. https://www.gs1.org/standards/barcodes/10-steps-to-barcode-your-product/english
Reference the Medicines Barcoding and Serialization Guideline from the National Health Regulatory Authority (NHRA) to understand the expectations for meeting barcoding and labeling medicines sold in Bahrain.
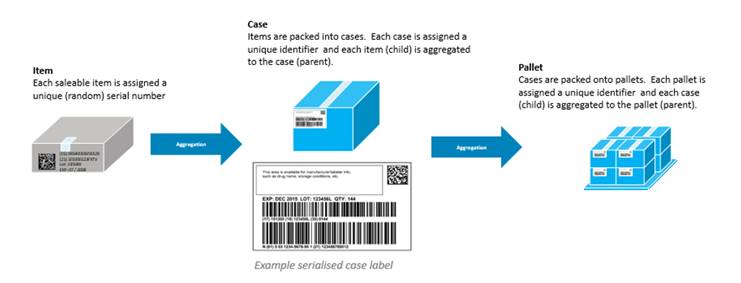
Aggregation defines the relationship between the parent and child barcodes allowing the receiver of the product to scan one code and understand exactly what is in the whole shipment — every case or individual carton. NHRA requires both the case/shipper and the pallet tertiary packaging aggregation barcodes & serialization.
Serialized Global Trade Identification Numbers (SGTIN) should be used on unit level medicines and for the case /shippers. Uniquely identified Serialized Shipping Container Code (SSCC) should be used for the pallet (tertiary level) aggregation barcodes & serialization.
NHRA requires both the secondary case/shipper and the pallet tertiary packaging aggregation barcodes & serialization.
Serialized Global Trade Identification Numbers (SGTIN) should be used on unit level medicines and for the case /shippers. Uniquely identified Serialized Shipping Container Code (SSCC) should be used for the pallet (tertiary level) aggregation barcodes & serialization.
All Invoicing Companies / MAHs must sign up with GS1 and have a Global Location Number (GLN) and Global Company Prefix (GCP) assigned. Make sure you know the GS1 Global Location Number (GLN) and Global Company Prefix (GCP) assigned to the manufacturing location of each product.
The NHRA-MVC Traceability Hub registration process for each company has 2 parts – the administrative registration and the technical onboarding.
The technical onboarding consists of
With confirmation and your successful registration and payment, the Administrative Contact and the Technical Point of Contact (T-POC) will receive an email stating the technical testing will be initiated.
