The National Health Regulatory Authority NHRA is a strong advocate of global standards for automatic identification of products and their manufacturers. Doing so, provides an opportunity to make the healthcare supply chain in the Kingdom of Bahrain safer as well as more efficient and accurate.
NHRA realized that the GS1 system of standards, which the global healthcare community has endorsed and is one of the most widely used trade item identification systems worldwide, should be implemented as the standard for international drug manufacturers exporting to the Kingdom of Bahrain. The NHRA is mandating the use of GS1 Data Matrix on the packaging of pharmaceutical products. Manufacturers and distributors are directed to the Medicines Barcoding and Serialization Guideline for the Kingdom of Bahrain to ensure consistency and traceability of products.
All products must be compliant with this new regulation by 31st December 2019. The benefits of its use for key stakeholders (manufacturers, distributors, importers, pharmacies and hospitals) is the ability for stakeholders to become compliant with the Supreme Council of Health resolution no. (41) for the year 2017 – Issuing the System for Tracking and Tracing Medicine Provision and Supply Chain inside the Kingdom of Bahrain.
The NHRA requires that all pharmaceutical manufacturers provide labeling for products that meet the GS1 defined standards. It is mandatory that all pharmaceutical trade items carry a 14-digit Global Trade Item Numbers (GTIN). As GS1 standards indicate, the use of Global Trade Item Numbers (GTINs) will help help industry make consistent decisions about the unique identification of pharmaceutical items throughout the supply chain. A sample representation of 14-digit GTINs is shown here:
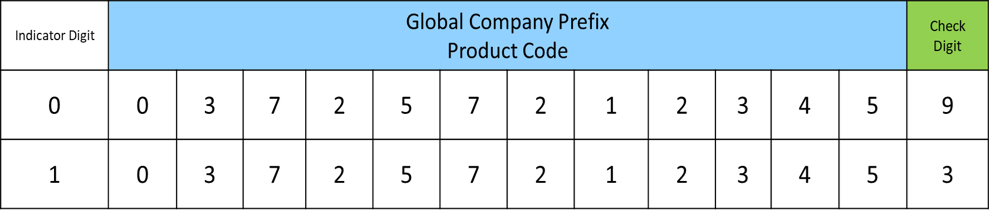
Unique identification of trade items through the use of GTINs is critical to maintaining operational efficiencies that business partners rely on to exchange information about products in consistent ways, as well as ensuring the smooth operations of global supply chains. GTINs provides unique identification for Healthcare Items. When combined with other relevant information such as the item’s batch number and expiration date, it will provide traceability from production to patient delivery.
Please reference the GS1 Healthcare GTIN Allocation Rules for detailed information regarding compliance with GTIN standards that will meet the NHRA requirements.
Barcodes are symbols that can be scanned electronically using laser or camera-based systems. Companies use them to encode information such as product numbers, serial numbers and batch numbers. Barcodes play a key role in pharmaceutical supply chains, enabling manufacturers, transport providers and hospitals to automatically identify and track products as they move through the supply chain.
The GS1 standards for barcoding of packages at the individual packaging level (GTIN) requires that both a 2D scannable label and information that can be read by a person, must be present. The Human readable version can carry the Application Identifier (AI), a field of two or more digits at the beginning of an element string that uniquely defines its format and meaning. All unit level barcoding must have the following attributes:
An identification key that uniquely identifies products worldwide. It can be encoded in various types of data carriers, including GS1 Data Matrix. A company receives a GS1 Company Prefix by joining a GS1 Member Organization. This gives the company the ability to create GTINs and access to the GS1 standards. You can refer to the GS1 General Specifications and GS1 Healthcare GTIN Allocation Rules for the GTIN Structure.
If you require additional data to identify an Expiration Date the GS1 Application Identifier (17) (often referred to as expiry date, use by date or maximum durability date) is used. It is expressed as year, month and day (YYMMDD).
Note: The Expiration Date data string can only specify dates within a certain range. Please refer to the GS1 General Specifications for additional information on structure and range
If Healthcare products are to be individually tracked and traced using a Serial Number, Application Identifier (21) can be used. This additional data is alphanumeric with a variable length of up to 20 characters. The SN determined by the pharmaceutical company does not need for a third party to get the SN. The GS1 application identifier used to identify the SN is 21.
If you require additional data to identify Batch or Lot Number, the GS1 Application Identifier (10) is used and typically assigned at the point of manufacture. This additional data is alphanumeric with a variable length of up to 20 characters. Please note that the above attributes are not standalone attributes and must be associated with a GTIN and encoded within the same Data Carrier.
Human readable interpretation (HRI) is the information below, beside or above a barcode which is encoded in the barcode and represents the same characters as carried in the barcode. Healthcare products often encounter regulatory, space, and technical constraints, so some specific rules are outlined within the GS1 General Specifications for these products.
The GS1 General; Specifications allow label designers to minimize space requirements through merging HRI and non-HRI text when meeting the GS1 recommended format is not practical. Doing so enables the manufacturer to display product safety information such as the batch / lot and expiry date, without the need to display it both as HRI and in a standard label structure.
The figures below illustrate one application of this approach, combining the data labels (e.g. GTIN, SN, and LOT, and expiry date) with application identifier (AI) labels (01, 17, 10, 21). This approach enables the label designer to simultaneously meet the HRI labelling requirements without having to print this information in two locations. Since the application identifier label is displayed next to each attribute, the data printed must follow the HRI rules, matching the data encoded in the barcode symbol.
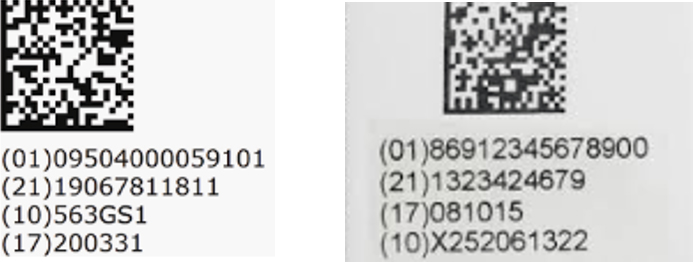
When the data format required to be displayed matches the HRI format, the AI may be used. However, when it does not match, the AI must be omitted, but the data label should be used to identify the attribute (refer to the GS1 General Specifications for more detail).
This applies to all encoded attributes that may appear on a healthcare product label. In the following examples, some data is shown in HRI, while other data is in non-HRI, When the non-HRI format is used, but the application identifier label, (17), is omitted. These label formats used in the examples are not intended to be descriptive or recommendations for meeting the requirements of the standards or local regulations.

If you are developing your artwork for packaging and still need to acquire your barcodes from GS1 you can find additional help for setting up your GS1 standard barcodes using 10-steps-to-barcode-your-product found in the GS1 library.
NHRA requires both the case/shipper and the pallet tertiary packaging aggregation barcodes & serialization. Aggregation defines the relationship between the parent and child barcodes allowing the receiver of the product to scan one code and understand exactly what is in the whole shipment — every case or individual carton. The tertiary labeling known as Serialized Shipping Container Codes (SSCC) is designed so that scanning the SSCC label will indicate what is contained within the case of pallet. This example indicates a typical parent child relationship for barcoding.
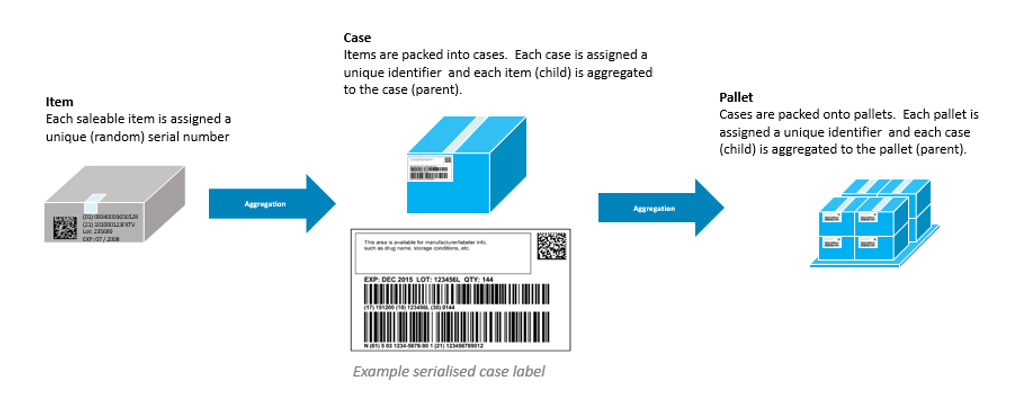
GS1 provides guidelines for aggregating from individual units to higher-level parent containers.
For which there is a need to retrieve predefined information (i.e. master data) and that may be priced, or ordered, or invoiced at any point in any supply chain.
Established for transport and/or storage that needs to be managed through the supply chain. Logistics units take many forms (e.g., a single box containing a limited number of products, a pallet of multiple products or an intermodal container containing multiple pallets).
You can find additional information regarding labeling and barcodes on the GS1 Website: GS1 Standards in Barcoding.