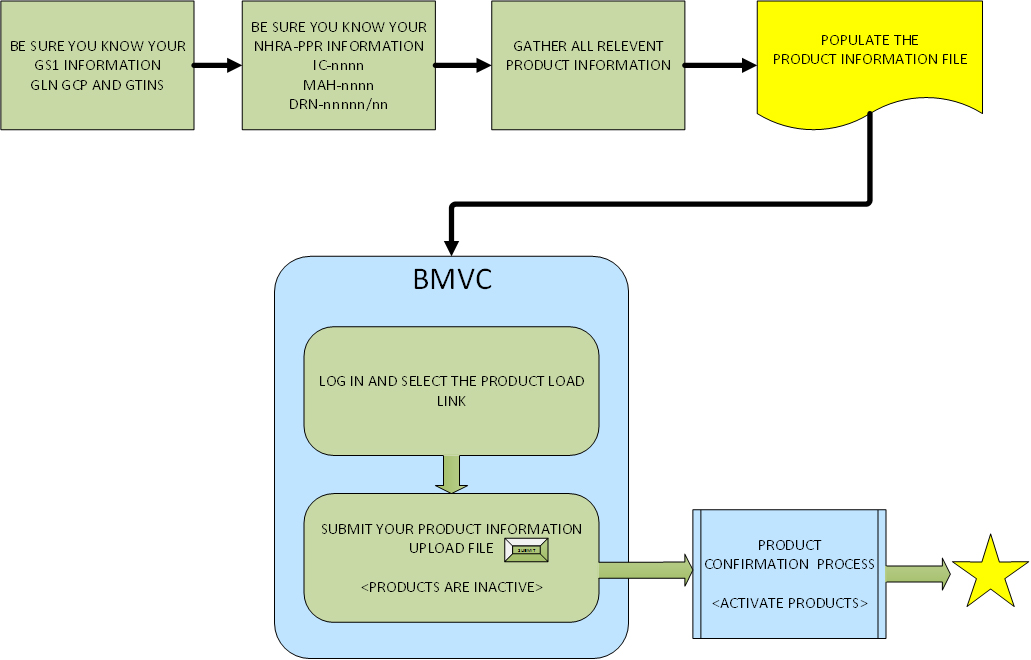
The MAH must load their product master data to the NHRA-MVC Traceability Hub. There are 6 steps for the MAH to be able to submit master data to the NHRA-MVC Traceability Hub. The first five steps are pre-requisites and must be completed before loading product information to the NHRA-MVC Traceability Hub.
To register products with the NHRA-MVC Traceability Hub the MAH will need to identify all products by their assigned Global Trade Identification Numbers (GTINs) and their manufacturing sites must be identified using properly formatted GS1 identifiers. All MAHs must be registered with GS1 and have a Global Location Number (GLN) and Global Company Prefix (GCP) assigned. Make sure you know the GS1 Global Location Number (GLN) and Global Company Prefix (GCP) assigned to the manufacturing location of each product.
Registration applications must be submitted and approved by the NHRA to permit pharmaceutical products to be sold in Bahrain. Payment must be made to the NHRA at the time of registration. The NHRA registration process contains all of the necessary MAH location information.
All MAH’s must complete a Participation Agreement contract with the NHRA prior to loading the product data.
Once the MAH has completed its NHRA and GS1 registrations, the Administrative Contact must select the Registration Link for MAH Registration. Your Participation Agreement contract (STEP 3 above) must be fully executed before the registration will be finalized.
To register your products with NHRA-MVC the MAH must first upload the list of manufacturing locations to the NHRA-MVC Traceability Hub so they can be linked to the products they will manufacture.
Once the MAH has completed Steps 1 through 5, products must be uploaded to the NHRA-MVC Traceability Hub. The MAH must upload the products to the NHRA-MVC Traceability Hub to complete the registration. The Technical Point of Contact should use the Master Data Load process to load the products for the MAH. The Technical POC will log into the NHRA-MVC website and select the Load Products link.
| Information Needed | Comments |
|---|---|
| Organization | Company Name as registered with the NHRA as the Invoicing Company |
| Invoicing Company Code (IC) | Assigned by the NHRA to the Invoicing Company (IC-nnnn) |
| Manufacturer (MAH) | Company Name registered with the NHRA and as the MAH. Will also be registered in the NHRA-MVC Traceability Hub as the MAH. |
| MAH Code | The code assigned to the Marketing Authorization Holder by the NHRA (MAH-nnnn) |
| Product Name | Medicine Name given to the NHRA-PPR when registering the product |
| Product ID | This is the Drug Registration Number (DRN) known to the NHRA-PPR (DRN-nnnnn/nn) |
| Alternate Product ID | If the permanent DRN has not been assigned, you must enter the Temporary Drug Number (TRN) assigned. |
| Product Code | This is the GTIN 14 assigned to the product in compliance with GS1 standards and provided to the NHRA-PPR. |
| Global Company Prefix Length | This is the GCP for this product and is required to format the full GS1 Unique Identifier properly |
| Strength | As registered with the NHRA-PPR for Strength and Unit of Strength (i.e. 5ml or 100IU/ML) |
| Dosage Form | As registered with the NHRA-PPR as the Pharmaceutical Form (i.e. Tablet, Cream, Powder, Syrup, Solution for Injection into Cartridge) |
| Product Type | Use DRUG for the GTIN-14 medicines. Use AGGREGATE for all secondary packaging (GS1 18-digit SSCC identifiers) |
| Container | Unit size and type (1 Case, 1 Pallet, 1 carton) |
| NHRA Drug Type |
Select either
|
| PPR Method of Sale |
Select the Method as registered with the NHRA-PPR
|
Each successfully loaded product will be initially set to INACTIVE status until the verification process can be completed. The NHRA-MVC Administrative team will verify your company and confirm the intended products are in good standing with the PPR.
With successful confirmation the status of the products will be set to ACTIVE in the NHRA-MVC Traceability Hub.