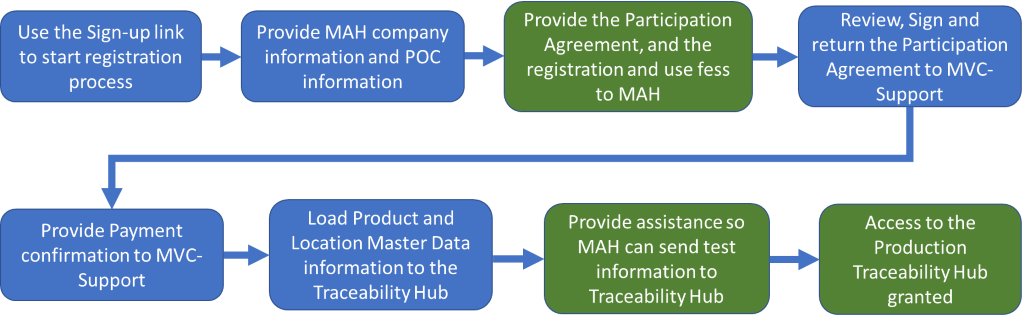
Each Marketing Authorization Holder (MAH) must register its own information in the NHRA-MVC Traceability Hub, sign the Participation Agreement and pay the required registration and use fees.
Before you sign up – The MAH must be sure they have been assigned MAH-CODE and their pharmaceutical products are accurately registered in the SEHATI Portal
- To find the pharmaceutical products that are registered to the MAH and the assigned MAH-CODE in the SEHATI Portal you can follow these instructions: Drug Look Up Instructions
Where to Start – Click the Sign-up link in the top navigation bar and select MAH to get started.
- Each MAH will provide information to identify their organization, their authorized Point of Contact (POC) and their Technical Point of Contact. The POC will receive the Participation Agreement to review, sign and return; and instructions for payment of the Sign up and Use Fees.
- After the MAH returns the Participation Agreement and has paid the required fees, the NHRA-MVC Technical team will assist the MAH to load their required Product and Location Master Data into the Traceability Hub.
Technical Onboarding – Each MAH will need to complete the Technical Onboarding process to certify they are prepared to send the proper transactions to the NHRA-MVC Traceability Hub.
- The MAH will send a series of information records (Transactions) to the Traceability Hub that represent actions commonly occurring in the manufacturing and shipping steps of the supply chain commissioning of serialized medicines, aggregation of cartons to cases, then the cases to pallets, and shipping the products to Bahrain.